RARE EARTH ELEMENTS: ABUNDANCES
“Rare” earth elements is a historical misnomer; persistence of the term reflects unfamiliarity rather than true rarity. The more abundant REE are each similar in crustal concentration to commonplace industrial metals such as chromium, nickel, copper, zinc, molybdenum, tin, tungsten, or lead (fig. 4). Even the two least abundant REE (Tm, Lu) are nearly 200 times more common than gold. However, in contrast to ordinary base and precious metals, REE have very little tendency to become concentrated in exploitable ore deposits. Consequently, most of the world’s supply of REE comes from only a handful of sources.
Differences in abundances of individual REE in the upper continental crust of the Earth (figs. 3, 4) represent the super-position of two effects, one nuclear and one geochemical. First, REE with even atomic numbers (58Ce, 60Nd, …) have greater cosmic and terrestrial abundances than adjacent REE with odd atomic numbers (57La, 59Pr, …). Second, the lighter REE are more incompatible (because they have larger ionic radii) and therefore more strongly concentrated in the continental crust than the heavier REE. In most rare earth deposits, the first four REE—La, Ce, Pr, and Nd—constitute 80 to 99% of the total. Therefore, deposits containing relatively high grades of the scarcer and more valuable heavy REE (HREE: Gd to Lu, Y) and Eu are particularly desirable.

Figure 3. Prices and abundances of the rare earth elements. Prices are for 1999 or 2000 in U.S. dollars per kilogram of REE metal, in two forms: (1) as oxides, in 2 to 25 kg packages, at 95 to 99.99% purity; (2) as 0.1 to 0.45 kg metal ingot, at 99.9% purity. Two representative REE ores—high-grade carbonatite ore from Mountain Pass, California, and lateritic ion-adsorption ore from southern China—are compared with Earth’s upper continental crust. Z, atomic number.
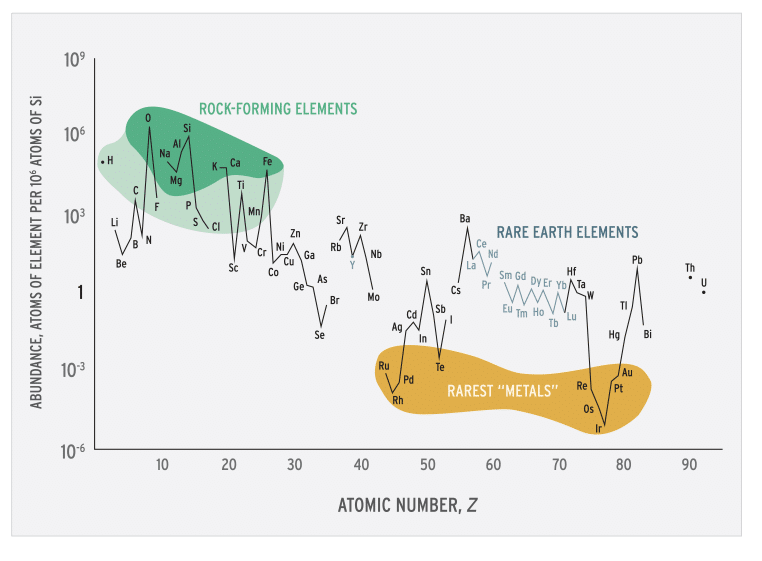
Figure 4. Abundance (atom fraction) of the chemical elements in Earth’s upper continental crust as a function of atomic number. Many of the elements are classified into (partially overlapping) categories: (1) rock-forming elements (major elements in green field and minor elements in light green field); (2) rare earth elements (lanthanides, La–Lu, and Y); (3) major industrial metals (global production >~3×107 kg/year); (4) precious metals (italic); and (5) the nine rarest “metals”—the six platinum group elements plus Au, Re, and Te (a metalloid).

From the discovery of the REE (during the period 1794–1907) through the mid-1950s, a few of the REE were produced in modest amounts from monazite-bearing placers and veins, from pegmatites and carbonatites, and as minor byproducts of uranium and niobium extraction. During this time, the middle and heavy REE generally were available in pure form only in sub-kilogram quantities and were chiefly chemical curiosities.
In 1949, a carbonatite intrusion with extraordinary contents of light REE (8 to 12% rare earth oxides [REO]), was discovered at Mountain Pass, in the upper Mojave Desert, California (fig. 5). The REE at Mountain Pass are hosted chiefly by bastnäsite, (Ce,La,Nd,…)CO3F, and related minerals. By 1966, this single, world-class deposit (owned and operated by Molycorp, Inc.) had become the paramount source of REE. Early development was supported largely by the
sudden demand for Eu created by the commercialization of color television. Mountain Pass, with an average grade of 9.3% and reserves of 20 million metric tons (Mt) REO (at 5% cutoff), remains the only large ore deposit mined solely for its REE content. Mountain Pass ore is very strongly dominated by the light REE (figs. 3, 6). Nonetheless, the large quantities of ore processed and development of solvent-extraction techniques for large-scale separation of individual REE from one another allowed recovery of several of the middle REE. Increased availability led in turn to applications for these formerly exotic elements.
From 1965 through the mid-1980s, Mountain Pass was the dominant source of REE, and the United States was largely self-sufficient in REE. Since 1985, production of REE in China has increased dramatically (fig. 1). Chinese REE production comes chiefly from two sources. The most important is the Bayan Obo iron-niobium-REE deposit, Inner Mongolia. This deposit has geological affinities both to carbonatite REE deposits and to hydrothermal iron-oxide (-Cu-Au-REE) deposits, such as Olympic Dam, Australia, and Kiruna, Sweden. Grades at Bayan Obo are 3 to 6% REO; reserves are at least 40 Mt, possibly considerably more. The second major source of Chinese REE is ion-adsorption ores in lateritic weathering crusts developed on granitic and syenitic rocks in tropical southern China. These oxide ores are advantageous in their relatively high proportions of HREE (fig. 6) and, especially, in the ease with which they can be mined and the REE extracted.
The number of workable REE deposits, already severely limited by the geochemical properties of the REE, has in recent years also been affected by environmental and regulatory factors. Monazite, the single most common REE mineral, generally contains elevated levels of thorium. Although Th itself is only weakly radioactive, it is accompanied by highly radioactive intermediate daughter products, particularly radium, that can accumulate during processing. Concern about radioactivity hazards has now largely eliminated monazite as a significant source of REE and focused attention on those few deposits where the REE occur in other, low-Th minerals, particularly bastnäsite.
