Rare Earth Elements: Critical Resources for High Technology
High-technology and environmental applications of the rare earth elements (REE) have grown dramatically in diversity and importance over the past four decades. As many of these applications are highly specific, in that substitutes for the REE are inferior or unknown, the REE have acquired a level of technological significance much greater than expected from their relative obscurity. Although actually more abundant than many familiar industrial metals, the REE have much less tendency to become concentrated in exploitable ore deposits. Consequently, most of the world’s supply comes from
only a few sources. The United States once was largely self-sufficient in REE, but in the past decade has become dependent upon imports from China.
The rare earth elements (REE) form the largest chemically coherent group in the periodic table. Though generally unfamiliar, the REE are essential for many hundreds of applications. The versatility and specificity of the REE has given them a level of technological, environmental, and economic importance considerably greater than might be expected from their relative obscurity. The United States once was largely self-sufficient in these critical materials, but over the past decade has become dependent upon imports (fig. 1). In 1999 and 2000, more than 90% of REE required by U.S. industry came from deposits in China.
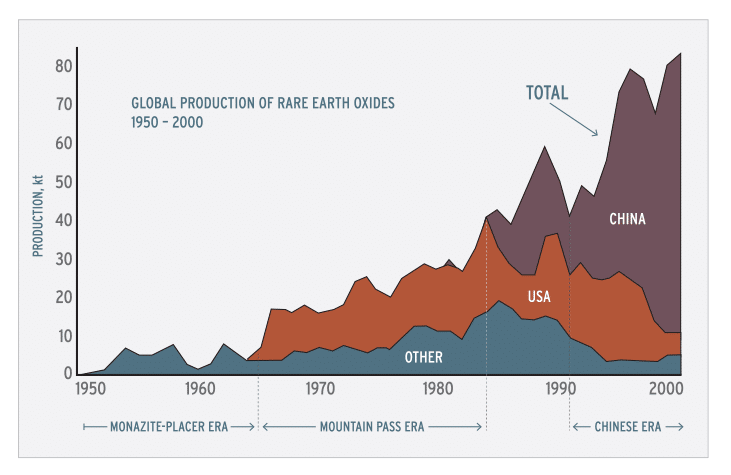
Figure 1. Global rare earth element production (1 kt=106 kg) from 1950 through 2000, in four categories: United States, almost entirely from Mountain Pass, California; China, from several deposits; all other countries combined, largely from monazite-bearing placers; and global total. Four periods of production are evident: the monazite-placer era, starting in the late 1800s and ending abruptly in 1964; the Mountain Pass era, starting in 1965 and ending about 1984; a transitional period from about 1984 to 1991; and the Chinese era, beginning about 1991.
Although the 15 naturally occurring REE (table 1; fig. 2) are generally similar in their geochemical properties, their individual abundances in the Earth are by no means equal. In the continental crust and its REE ore deposits, concentrations of the most and least abundant REE typically differ by two to five orders of magnitude (fig. 3). As technological applications of REE have multiplied over the past several decades, demand for several of the less abundant (and formerly quite obscure) REE has increased dramatically.
Names and Symbols of The Ree
| La | Lanthanum | Tb | Terbium |
| Ce | Cerium | Dy | Dysprosium |
| Pr | Praseodymium | Ho | Holmium |
| Nd | Neodymium | Er | Erbium |
| Pm | Promethium | Tm | Thulium |
| Sm | Samarium | Yb | Ytterbium |
| Eu | Europium | Lu | Lutetium |
| Gd | Gadolinium | Y | Yttrium |
Table 1. The diverse nuclear, metallurgical, chemical, catalytic, electrical, magnetic, and optical properties of the REE have led to an ever increasing variety of applications. These uses range from mundane (lighter flints, glass polishing) to high-tech (phosphors, lasers, magnets, batteries, magnetic refrigeration) to futuristic (high-temperature superconductivity, safe storage and transport of hydrogen for a post-hydrocarbon economy).

Figure 2. Chemical periodic table delineating the 16 rare earth elements (REE): the lanthanides, La through Lu, plus Y, whose geochemical behavior is virtually identical to that of the heavier lanthanides. Promethium has no long-lived isotopes and occurs naturally on Earth only in vanishingly small quantities. An represents the first 14 actinide elements; Lr is the last actinide.
