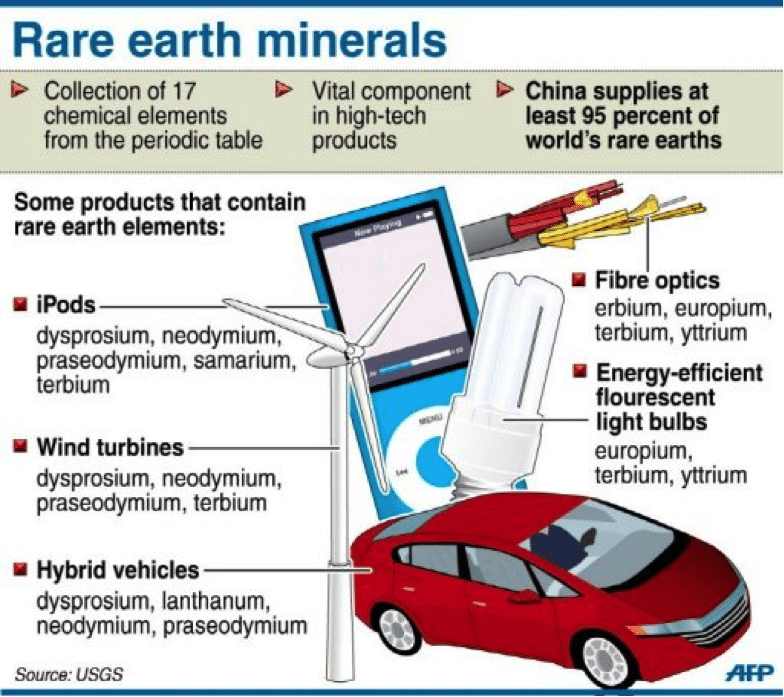
Rare Earth Elements: Applications
Many applications of REE are characterized by high specificity and high unit value. For example, color cathode-ray tubes and liquid-crystal displays used in computer monitors and televisions employ europium as the red phosphor; no substitute is known. Owing to relatively low abundance and high demand, Eu is quite valuable—$250 to $1,700/kg (for Eu2O3) over the past decade.
Fiber-optic telecommunication cables provide much greater bandwidth than the copper wires and cables they have largely replaced. Fiber-optic cables can transmit signals over long distances because they incorporate periodically spaced lengths of erbium-doped fiber that function as laser amplifiers. Er is used in these laser repeaters, despite its high cost (~$700/kg), because it alone possesses the required optical properties.
Specificity is not limited to the more exotic REE, such as Eu or Er. Cerium, the most abundant and least expensive REE, has dozens of applications, some highly specific. For example, Ce oxide is uniquely suited as a polishing agent for glass. The polishing action of CeO2 depends on both its physical and chemical properties, including the two accessible oxidation states of cerium, Ce,3+ and Ce4+, in aqueous solution. Virtually all polished glass products, from ordinary mirrors and eyeglasses to precision lenses, are finished with CeO2.
Permanent magnet technology has been revolutionized by alloys containing Nd, Sm, Gd, Dy, or Pr. Small, lightweight, high-strength REE magnets have allowed miniaturization of numerous electrical and electronic components used in appliances, audio and video equipment, computers, automobiles, communications systems, and military gear.
Many recent technological innovations already taken for granted (for example, miniaturized multi-gigabyte portable disk drives and DVD drives) would not be possible without REE magnets.

Currently, some of these REE applications have no substitutes. The rare earth elements are essential for a diverse and expanding array of high-technology applications, which constitute an important part of the industrial economy of the United States. Long-term shortage or unavailability of REE would force significant changes in many technological aspects of American society.
Environmental applications of REE have increased markedly over the past three decades. This trend will undoubtedly continue, given growing concerns about global warming and energy efficiency. Several REE are essential constituents of both petroleum fluid cracking catalysts and automotive pollution-control catalytic converters. Use of REE magnets reduces the weight of automobiles. Widespread adoption of new energy-efficient fluorescent lamps (using Y, La, Ce, Eu, Gd, and Tb) for institutional lighting could potentially achieve reductions in U.S. carbon dioxide emissions equivalent to removing one-third of the automobiles currently on the road. Large-scale application of magnetic-refrigeration technology (described below) also could significantly reduce energy consumption and CO2 emissions.
In many applications, REE are advantageous because of their relatively low toxicity. For example, the most common types of rechargeable batteries contain either cadmium (Cd) or lead. Rechargeable lanthanum-nickel-hydride (La-Ni-H) batteries are gradually replacing Ni-Cd batteries in computer and communications applications and could eventually replace lead-acid batteries in automobiles. Although more expensive, La-Ni-H batteries offer greater energy density, better charge-discharge characteristics, and fewer environmental problems upon disposal or recycling. As another example, red and red-orange pigments made with La or Ce are superseding traditional commercial pigments containing Cd or other toxic heavy metals.

The next high-technology application of the REE to achieve maturity may be magnetic refrigeration. The six REE ions Gd3+ through Tm3+ have unusually large magnetic moments, owing to their several unpaired electrons. A newly developed alloy, Gd5(Si2Ge2), with a “giant magne-tocaloric effect” near room temperature reportedly will allow magnetic refrigeration to become competitive with conventional gas-compression refrigeration. This new technology could be employed in refrigerators, freezers, and residential, commercial, and automotive air conditioners. Magnetic refrigeration is considerably more efficient than gas-compression refrigeration and does not require refrigerants that are flammable or toxic, deplete the Earth’s ozone layer, or contribute to global warming.
